Home / About Blue-Light Cystoscopy
About Blue-Light Cystoscopy
Cystoscopy is used to inspect your bladder lining for abnormal growth or suspicious tissue. It may be used to help find the cause of symptoms or to monitor changes. Based on findings of the cystoscopy, suspicious tissue may be removed for proper testing. The standard is to perform the procedure using white light only (also known as White-Light Cystoscopy or WLC).
What is Blue-Light Cystoscopy?
Since abnormal tissue can be difficult to detect, a new enhanced imaging agent is used to help improve detection of non-muscle invasive bladder cancer (NMIBC). The imaging agent is instilled into your bladder and is preferentially absorbed by abnormal tissue. Your bladder is then examined using blue light (also known as Blue-Light Cystoscopy or BLC) which makes abnormal tissue illuminate pink against a dark blue background.
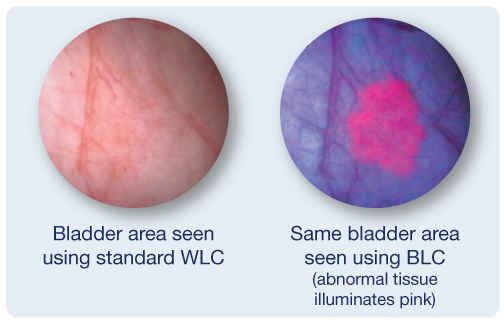
What are the Benefits of Using Blue-Light Cystoscopy?
Bladder cancer patients face a high risk for recurrence and progression into muscle-invasive bladder cancer.1 The risk of recurrence is due, in part, to incomplete surgeries (ie. missed tumours or tumours not fully removed).2,3
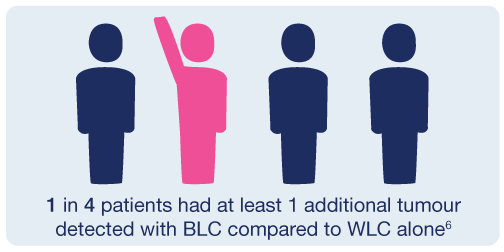
By using BLC for non-muscle invasive bladder cancer, physicians can see more tumours than with standard WLC alone.2,3,4,5
There are more than 80,000 Canadians living with bladder cancer and 9,000 Canadians will be newly diagnosed this year.7 This disease is not only an emotional and physical burden on patients and their caregivers, but also a financial strain on healthcare resources. Bladder cancer is the most expensive cancer to manage/treat (based on cumulative per patient basis),8 due to frequent follow ups and surgical procedures.
BLC aids physicians in performing thorough exams and more complete resections, which can help reduce the risk of recurrence and lessen the frequency at which require follow-up procedures.
Where is Blue-Light Cystoscopy Available?
BLC has been successfully used in Europe since 2008 and in the USA since 2011. In 2015, BLC was approved for use in Canada, yet it still is not widely available across the country (see below for recent news articles).
All patients deserve access to the same level of care. You can help urge your physician, hospital, and government elected officials to make this technology available for all appropriate bladder cancer patients.
Blue-Light Cystoscopy in the News:
- Toronto.com (Jan 30, 2017) – Donors help bring Cysview technology to Humber River Hospital’s urology department
- KGH Connect (Mar 24, 2017) – KGH first hospital in Canada to use technique outside of clinical trial to locate bladder cancer
- TheWhig.com (Apr 5, 2017) – Procedure improves quality of life for bladder cancer patients
- TorontoSun.com (Jan 25, 2018) – Humber Hospital has the pulse of the community
- PersonalHealthNews.ca (Mar 15, 2018) – New Tech Improving Outcomes for Bladder Cancer, but Barriers to Access Remain
- VancouverSun.com (Jun 1, 2018) – Vancouver urologists shine new light on bladder cancers to improve detection
References
- Sylvester RJ et al. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur Urol 2006; 49: 466-467
- Jocham D, Witjes F, Wagner S, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2005;174(3):862-866.
- Grossman HB, Gomella L, Fradet Y, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol. 2007;178(1):62-73.
- Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010;184(5):1907-1913.
- Hermann GG, Mogensen K, Carlsson S, Marcussen N, Duun S. Fluorescence-guided transurethral resection of bladder tumours reduces bladder tumour recurrence due to less residual tumour tissue in Ta/T1 patients: a randomized two-centre study. BJU Int. 2011;108(8 pt 2):E297-303
- Burger M et al. Photodynamic Diagnosis of Non–muscle-invasive Bladder Cancer with Hexaminolevulinate Cystoscopy: A Meta-analysis of Detection and Recurrence Based on Raw Data. European Urology 2013; EURURO-5062; No. of Pages 9.
- Bladder Cancer Facts. Bladder Cancer Canada. Web address: https://bladdercancercanada.org/en/bladder-cancer-facts/. Access date: Sept 24, 2018.
- CUA guidelines on the management of non-muscle invasive bladder cancer. Can Urol Assoc J 2015;9(9-10):E690-704. http://dx.doi.org/10.5489/cuaj.3320. Published online October 13, 2015.